
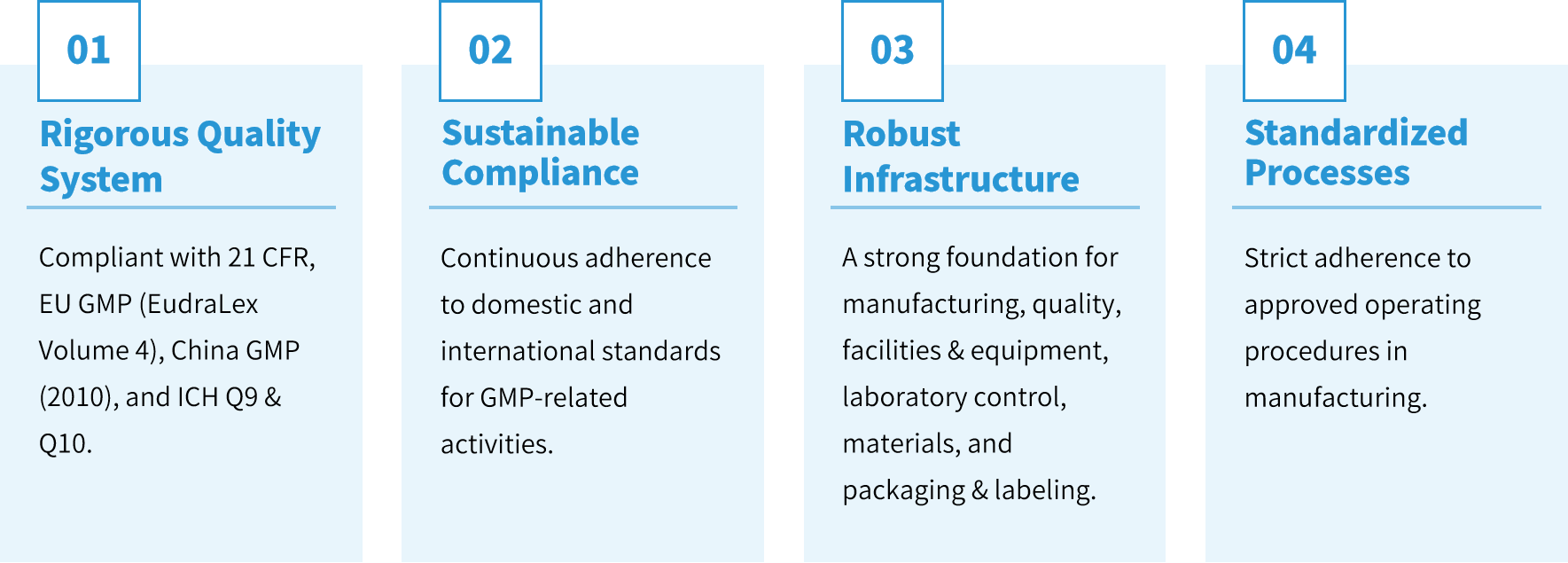
Genevoyager GMP Quality System
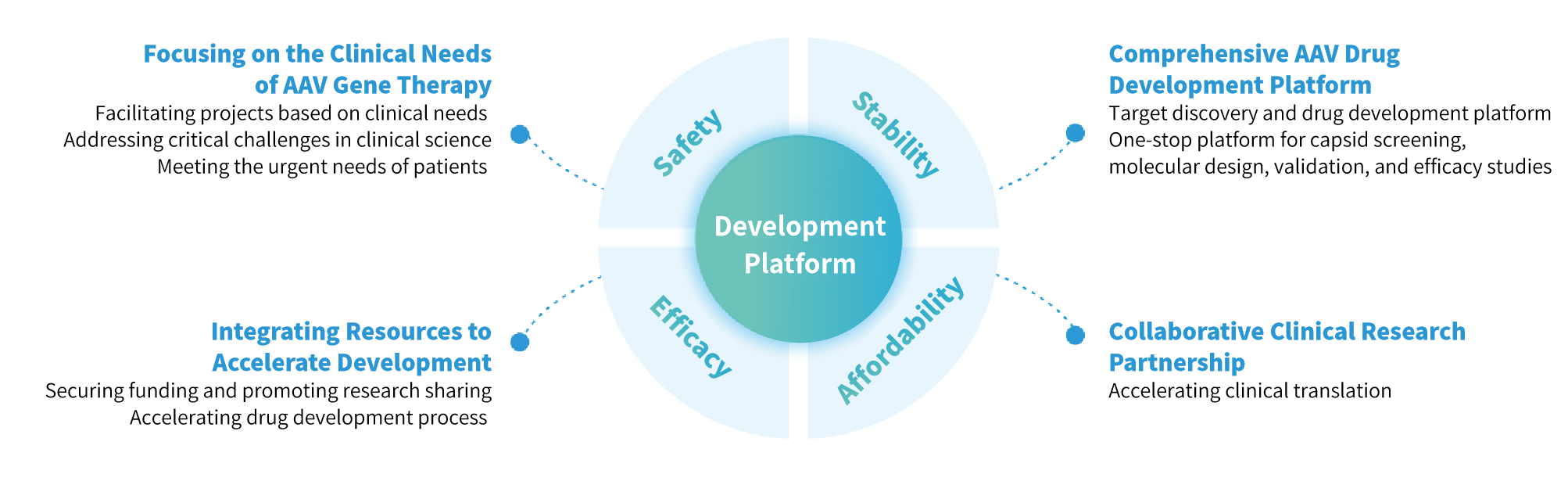
Genevoyager is committed to improving patient health by integrating clinical insights and addressing real-world needs. We focus on the clinical application of gene therapy, providing one-stop solutions - from research and validation to manufacturing - to meet the challenges faced by patients and healthcare providers.
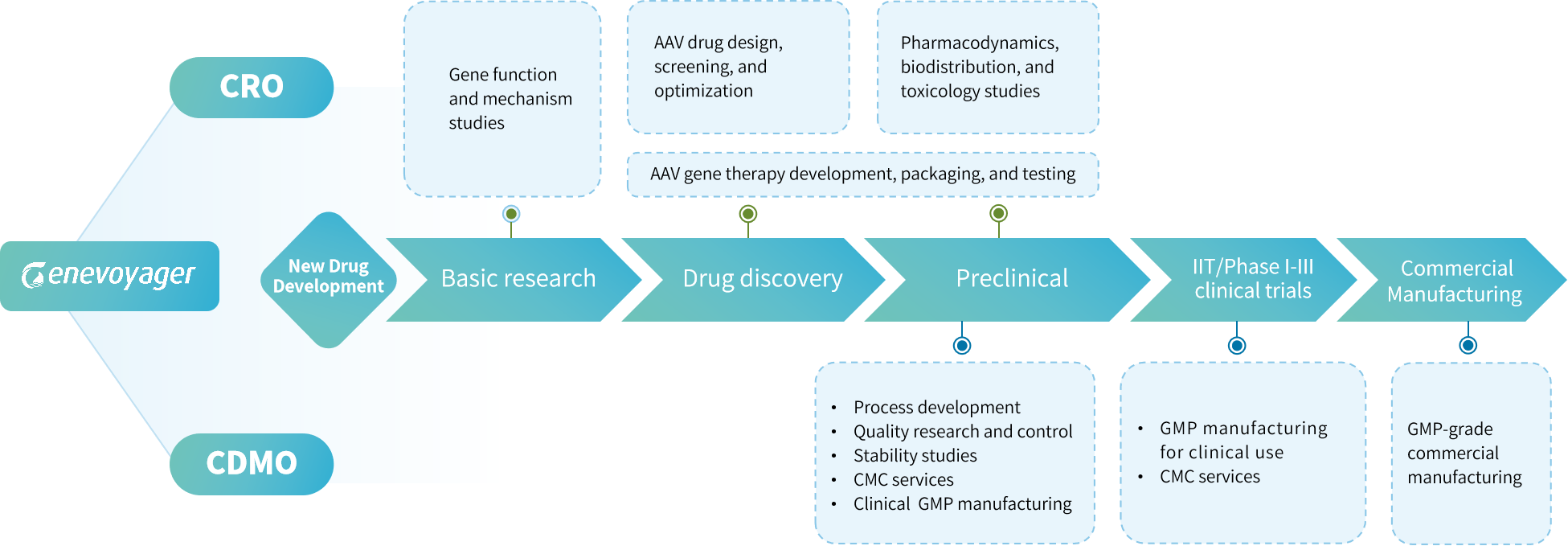
Drug Development
Genevoyager offers a well-established one-stop platform for AAV vector design, construction, packaging, and testing, empowering precise targeted therapy for diseases through various gene engineering technologies and administration routes.

Current Clinical Applications of Gene Therapy in Major Human Diseases
|
Category |
Disease |
|
Neurological disorders
|
Spinal Muscular Atrophy (SMA), Amyotrophic Lateral Sclerosis (ALS), Temporal Lobe Epilepsy (TLE), Frontotemporal Dementia (FTD), Huntington’s Disease (HD), Parkinson’s Disease (PD), Alzheimer’s Disease (AD), Neuronal Ceroid Lipofuscinosis (NCL), Batten Disease, Canavan Disease, Dravet Dyndrome (DS), Giant Axonal Neuropathy (GAN), GM1 Gangliosidosis, GM2 Gangliosidosis, Metachromatic Leukodystrophy (MLD), Multiple System Atrophy (MSA), CLN5-NCL, Spastic Paraplegia 50 (SPG50), etc. |
|
Neuromuscular diseases |
Limb-Girdle Muscular Dystrophy (LGMD), Duchenne Muscular Dystrophy (DMD), Charcot-Marie-Tooth (CMT), Becker Muscular Dystrophy (BMD), Friedreich Ataxia (FA), X-Linked Myotubular Myopathy (X-MTM), etc. |
|
Ocular diseases |
Leber Congenital Amaurosis (LCA), Leber Hereditary Optic Neuropathy (LHON), X-Linked Retinoschisis (XLRS), X-Linked Retinitis Pigmentosa (XLRP), CNGA3/CNGB3-mutated Achromatopsia (ACHM), Wet Age-related Macular Degeneration (Wet AMD), Dry Age-related Macular Degeneration (Dry AMD, map atrophy), Choroideremia (CHM), Retinitis Pigmentosa (RP), Stargardt Disease (STGD), etc. |
|
Metabolic diseases |
Mucopolysaccharidosis (MPS), Gaucher Disease (GD), Fabry Disease (FD), Glycogen Storage Disease (GSD), Aromatic L-Amino Acid Decarboxylase Deficiency (AADCD), Ornithine Transcarbamylase Deficiency (OTCD), Krabbe Disease (KD), Hepatolenticular Degeneration (HLD), Homozygous Familial Hypercholesterolaemia (HoFH), Familial Hypertriglyceridemia (FHTG), Familial Lipoprotein Lipase (LPL) Deficiency, Methylmalonic Acidemia (MMA), Phenylketonuria (PKU), Pompe Disease (PD), etc. |
|
Cardiovascular diseases |
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), Hypertrophic Cardiomyopathy (HCM), Chronic Heart Failure (CHF), Critical Limb Ischemia (CLI), Dilated Cardiomyopathy (DCM), Hereditary Angioedema (HAE), Familial Hypercholesterolemia (FH), Ischemic Cardiomyopathy (ICM), PKP2-related ARVC, etc. |
|
Hematologic diseases |
Hemophilia A (HA), Hemophilia B (HB), etc. |
|
Otologic disease |
Autosomal Recessive Deafness 9 (DFNB9), etc. |
|
Chronic inflammatory diseases |
Osteoarthritis (OA), etc. |
|
Cancer |
Gastric Cancer (GC), etc. |
Process Highlights
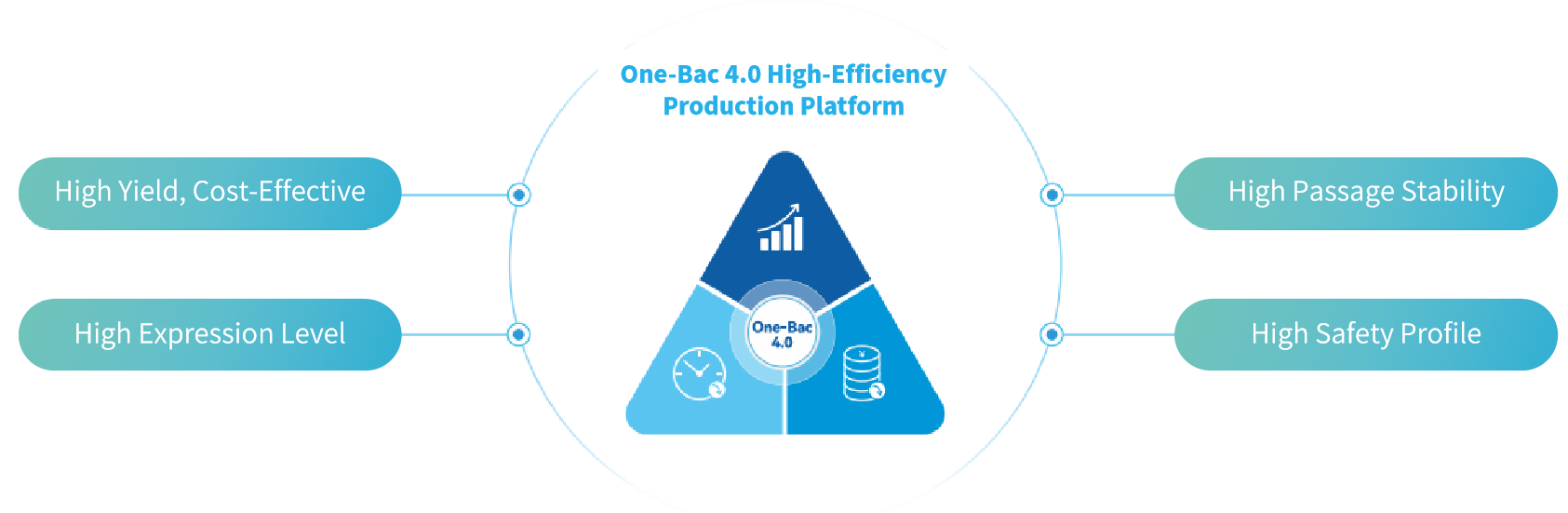
▎High Yield, Cost-Effective
The One-Bac 4.0 system ensures stable AAV yields across various production scales, with initial yields reaching up to 1~5E+15 vg/L and batch yields exceeding 1E+18 vg. Reduced manufacturing costs and higher production capacity facilitate meeting the medical needs of a broad patient population.
▎High Expression Level
Results from in vitro and in vivo experiments demonstrate that One-Bac 4.0-derived rAAV exhibits equivalent or higher expression level than HEK293 rAAV.
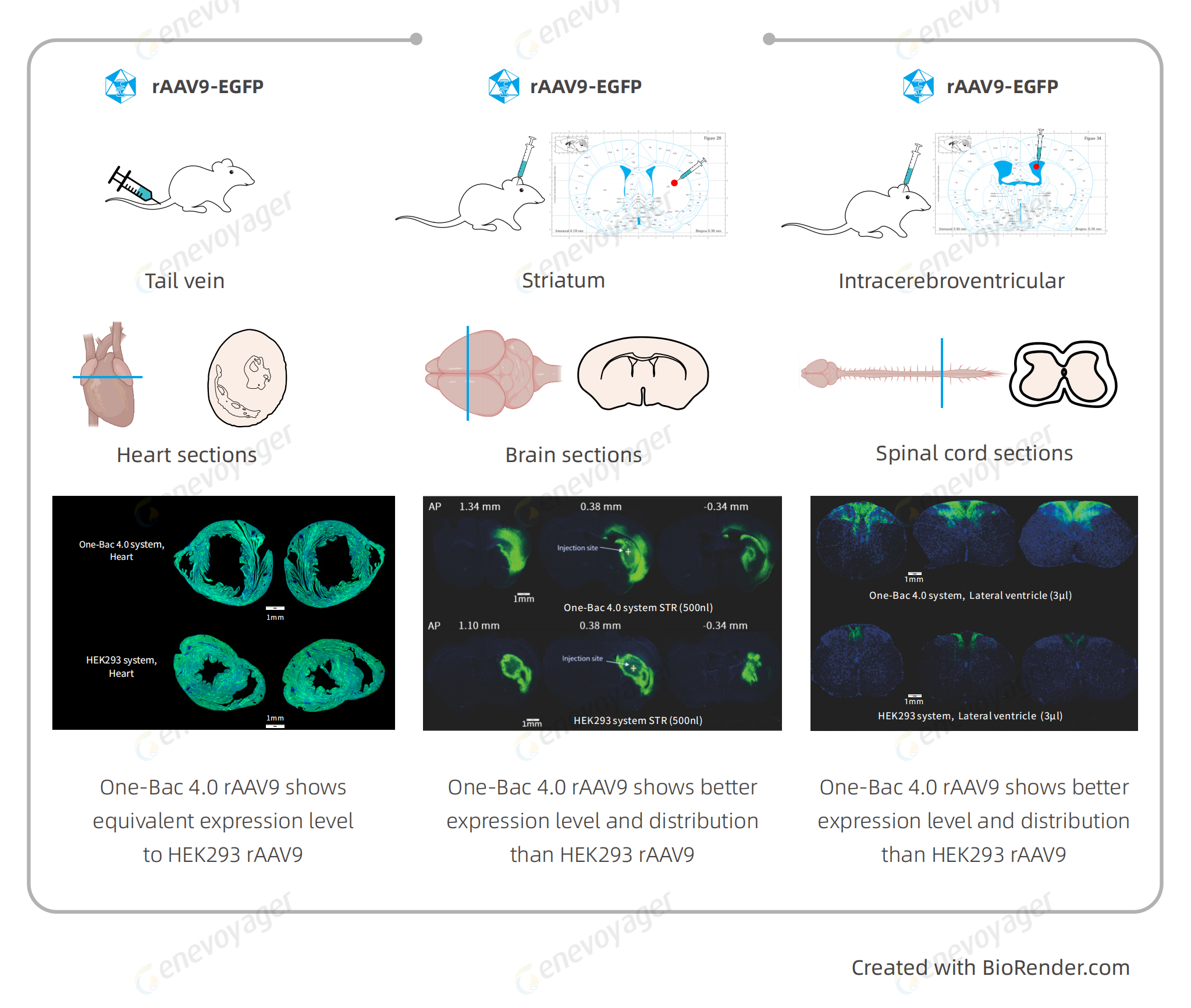
▎High Passage Stability
The Baculovirus Expression Vector (BEV) employed in the One-Bac 4.0 system maintains high stability for multiple passages, ensuring consistent high yield and expression level of AAV batches during large-scale production.
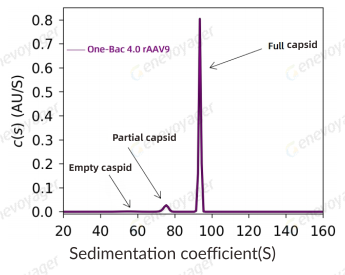
▎High Safety Profile
The AAV produced using the One-Bac 4.0 system features high full capsid ratio, minimal impurities, and is free of replication-competent AAV (rcAAV).

US: 3675 Market Street, Suite 200, Philadelphia, PA19104 Tel: +1 (215) 205-6963 | +086 027-65023363
E-mail: hui.wang@genevoyager.com
China: No128, Guanggu 7th Rd, East Lake High-tech Development Zone, Wuhan, China Tel: 17720522078
E-mail: marketing@genevoyager.com