One-Bac 4.0 system is an insect/baculovirus system for AAV production. The production process consists of several key parts: baculovirus plasmid construction, seed virus production, seed virus amplification, seed virus infection of insect cells, and AAV harvest. This system achieves linear amplification of production by infecting insect cells with baculovirus containing the AAV genome, overcoming the bottleneck of large-scale AAV preparation. One-Bac 4.0 system has the potential to benefit more patients due to its high yield compared to other AAV production systems.
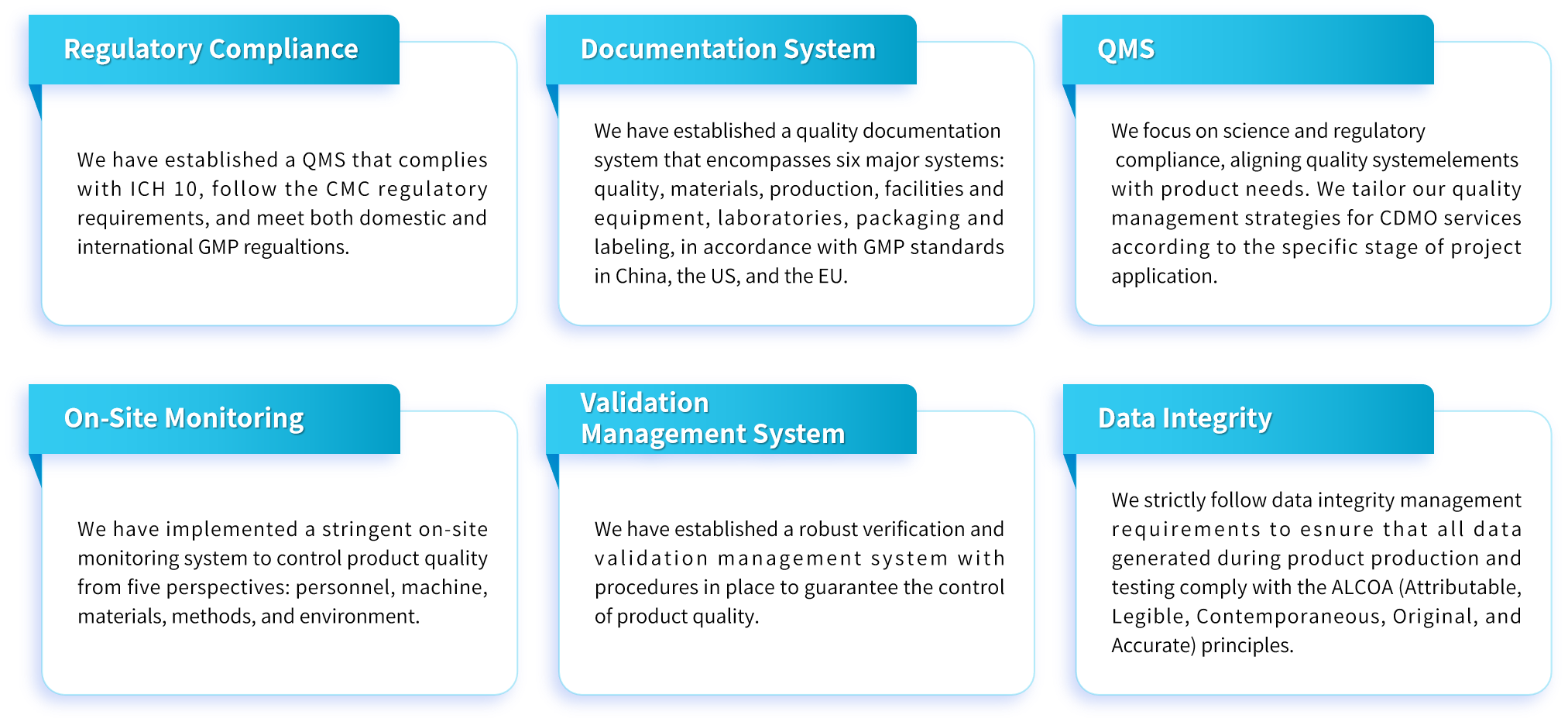
Genevoyager is equipped with a one-stop gene therapy CDMO platform, alongsidean experienced GMP quality control teamwithexpertise inmethodology development, validation/verification, and testing toensure the high quality of our products.The routine quality release tests and methods are outlined below.

US: 3675 Market Street, Suite 200, Philadelphia, PA19104 Tel: +1 (215) 205-6963 | +086 027-65023363
E-mail: hui.wang@genevoyager.com
China: No128, Guanggu 7th Rd, East Lake High-tech Development Zone, Wuhan, China Tel: 17720522078
E-mail: marketing@genevoyager.com