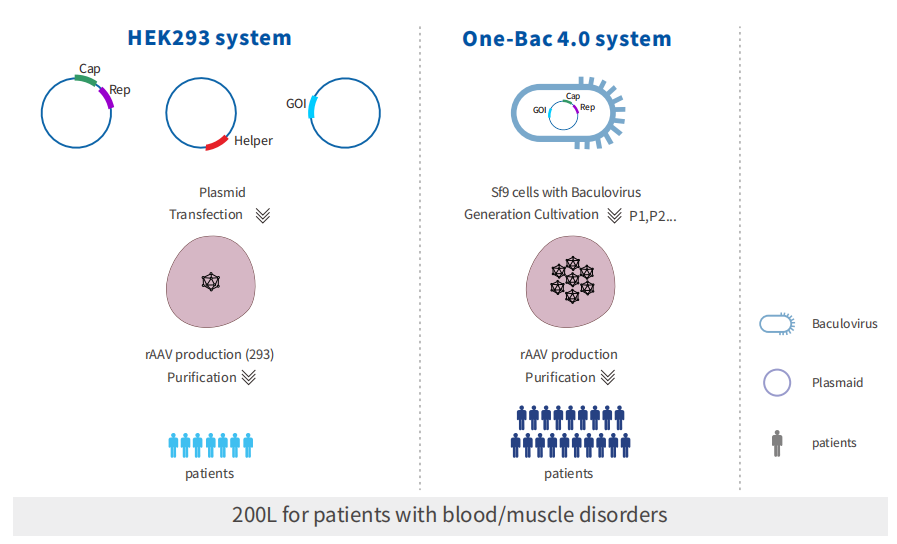
Unlike the traditional triple-plasmid system in HEK293 cells, the One-Bac 4.0 system employs an insect/baculovirus platform for AAV production. The workflow includes four key stages: baculovirus plasmid construction, virus seed generation and expansion,infection of insect cells, and AAV harvest. This system achieves linear amplification of production by infecting insect cells with baculovirus containing the AAV genome, overcoming the bottleneck of large-scale AAV manufacturing. With its higher yield compared to conventional approaches,the One-Bac 4.0 system has the potential to broaden patient access to AAV-based therapies.
Genevoyager has successfully completed multiple projects, with selected data presented below.
● High Safety Profile
AAVs produced using the One-Bac 4.0 system exhibit low impurity levels and are free of rcAAV.
Data from second-generation sequencing (illumina) and third-generation sequencing (PacBio) confirm the high purity of Genevoyager's One-Bac 4.0-derived AAVs.
The One-Bac 4.0 system ensures AAV production free of rcAAV.

DNA was extracted from the culture supernatant after three cell passages, and rcAAV was detected by qPCR. The results demonstrated:
For the positive control samples (wtAAV2/9) at 100 IU and 10 IU,Ct values decreased progressively across three amplification rounds, confirming rcAAV amplification during culture.
For rAAV2/9 produced using the One-Bac 4.0 system,at inoculation doses of 1E+11 vg and 1E+10 vg, no significant decrease in Ct values was observed after three passages.Ct values remained consistent with those of the negative control (NC), indicating the absence of detectable rcAAV.
In contrast,for rAAV2/9 produced using the HEK293T system at an inoculation dose of 1E+10 vg, Ct values demonstrated a clear decreasing trend across three amplification rounds,confirming rcAAV propagation during culture.
● High Yields
The One-Bac 4.0-derived AAVs consistenly achieve high yields both before and after purification at a production scale of 50L.

● High Full Capsid Ratio
The One-Bac 4.0 system delivers a final product with a full capsid ratio exceeding 90%.

|
Manufacturing system |
Product |
Purification method |
AUC% empty |
AUC% partial |
AUC% full |
AUC% aggregates |
|
Genevoyager HEK293 |
Final product |
Affinity+Anion Exchange |
7.52 |
0 |
>90 |
0.16 |
|
One-Bac 4.0 |
Final product |
Affinity+Anion Exchange |
1.1 |
7.2 |
>90 |
0 |
● High Infectious Activity
Results from cell and animal experiments demonstrate that One-Bac 4.0-derived AAV exhibits equivalent or higher infectivity compared to HEK293 AAV.

Comparison of firefly luciferase activity in HEK293 cells infected with AAV-Luc produced by the One-Bac 4.0 system and the HEK293 system at the same MOI.

Comparison of transduction efficiency of rAAV9-EGFP and rAAV9-mCherry produced by the One-Bac 4.0 system and the HEK293 system (physical titer/infectious titer, TCID50).
● High Passage Stability of BEV
The Baculovirus Expression Vector (BEV) used in the One-Bac 4.0 system demonstrates exceptional stability across multiple passages and after long-term storage at≤-80℃ for over 24 months.This ensures reliable large-scale manufacturing with consistently high AAV yields and robust expression levels suitable for commercial production.

● High Recovery Rate
The One-Bac 4.0 system delivers reliable scalability and efficiency for large-scale manufacturing, maintaining a consistent recovery rate of ≥30% from 5L to 200L scale-up.

● Low Aggregation at High Concentration
AAVs produced with the One-Bac 4.0 system show low aggregation even at high concentrations. Stability studies using ddPCR-GOl and SEC-HPLC confirm that product quality is preserved during storage at 4℃ and remains robust after multiple freeze-thaw cycles.
|
Stability at 4℃ and 27℃(AAV9) |
||||
|
Incubation Conditions |
Results |
|||
|
Temperature |
Days(D) |
ddPCR-GOI(vg/mL) |
Main peak SEC-HPLC(%) |
DLS |
|
Diameter(nm) |
||||
|
Control group |
0 |
2.76E+14 |
96.18 |
31.76 |
|
4℃ |
7 |
2.84E+14 |
95.80 |
33.20 |
|
14 |
2.69E+14 |
95.51 |
33.70 |
|
|
27℃ |
3 |
2.86E+14 |
95.91 |
31.11 |
|
7 |
2.49E+14 |
95.70 |
31.98 |
|
|
14 |
2.84E+14 |
94.60 |
30.32 |
|
|
Freeze-thaw Stability at-80℃(AAV9) |
|||
|
Processing Conditions |
Results |
||
|
ddPCR-GOI(vg/mL) |
Main peak SEC-HPLC(%) |
DLS |
|
|
Diameter(nm) |
|||
|
Control |
2.76E+14 |
96.18 |
31.76 |
|
3 Freeze-thaw cycles |
2.98E+14 |
95.05 |
31.15 |
|
5 Freeze-thaw cycles |
2.64E+14 |
94.12 |
32.12 |
● Improved Accessibility
The One-Bac 4.0 system provides a cost-effective solution for AAV production by replacing expensive GMP-grade plasmids and transfection reagents with a streamlined baculovirus-based process, significantly lowering material costs in vector manufacturing.
● Reduced timelines
A virus seed bank enables a rapid, streamlined infection process during production. This advantage becomes increasingly significant at larger scales, helping to shorten manufacturing timelines and accelerate delivery.
|
Packaging System
|
Phase Il (up to 50 patients) |
Phase Ⅲ
(up to 200 patients)
|
Commercial Stage
(up to 3,000 patients)
|
|
Genevoyager
One-Bac 4.0 system
|
Fermentation at 500L |
Fermentation at 2,000L |
Fermentation at 5,000L |
|
Purification at 500L |
Purification at 2,000L |
Purification at 5,000L |
|
|
Testing |
Testing |
Testing |
|
|
Estimated timeline |
10-12 weeks |
12-14 weeks |
14-15 weeks |
|
HEK293 serum-free
suspension system
|
Preparation of 3 plasmids |
—— |
—— |
|
Fermentation at 500L-1,000L |
—— |
—— |
|
|
Purification at 1,000L |
—— |
—— |
|
|
Testing |
—— |
—— |
|
|
Estimated timeline |
18-20 weeks |
Difficult to achieve |
Difficult to achieve |

US: 3675 Market Street, Suite 200, Philadelphia, PA19104 Tel: +1 (215) 205-6963 | +086 027-65023363
E-mail: hui.wang@genevoyager.com / vector@genevoyager.com
China: No128, Guanggu 7th Rd, East Lake High-tech Development Zone, Wuhan, China Tel: 17720522078
E-mail: marketing@genevoyager.com