Genevoyager has successfully completed multiple projects, with selected data presented below.
Genevoyager provides end-to-end solutions for gene therapy,backed by an experienced GMP quality control team with expertise in method development, validation/verification, and testing to ensure the consistent,highest quality of our products. A summary of quality release tests and their corresponding methodologies is provided below:
AAVs produced using the One-Bac 4.0 system exhibit low impurity levels and are free of rcAAV.
Data from second-generation sequencing (illumina) and third-generation sequencing (PacBio) confirm the high purity of Genevoyager's One-Bac 4.0-derived AAVs.
The One-Bac 4.0 system ensures AAV production free of rcAAV.
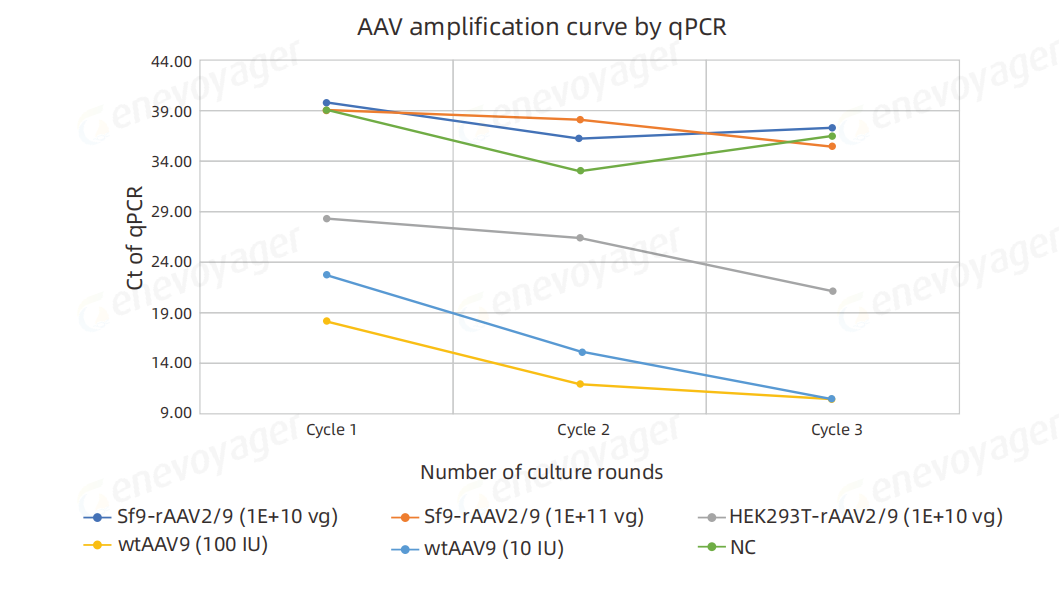
DNA was extracted from the culture supernatant after three cell passages, and rcAAV was detected by qPCR. The results demonstrated:
For the positive control samples (wtAAV2/9) at 100 IU and 10 IU,Ct values decreased progressively across three amplification rounds, confirming rcAAV amplification during culture.
For rAAV2/9 produced using the One-Bac 4.0 system,at inoculation doses of 1E+11 vg and 1E+10 vg, no significant decrease in Ct values was observed after three passages.Ct values remained consistent with those of the negative control (NC), indicating the absence of detectable rcAAV.
In contrast,for rAAV2/9 produced using the HEK293T system at an inoculation dose of 1E+10 vg, Ct values demonstrated a clear decreasing trend across three amplification rounds,confirming rcAAV propagation during culture.
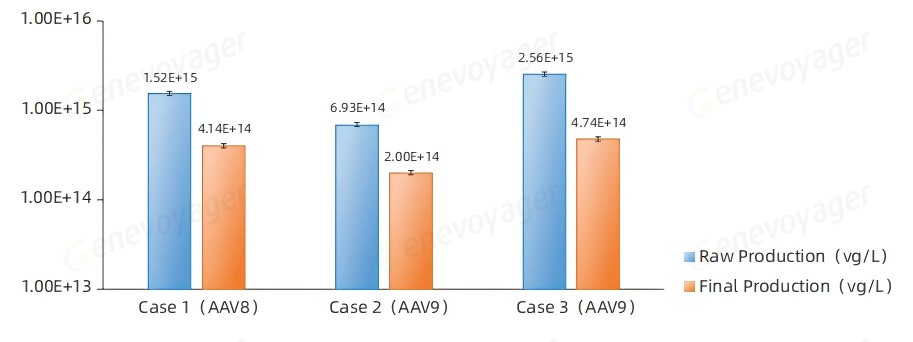
The One-Bac 4.0-derived AAVs consistenly achieve high yields both before and after purification at a production scale of 50L.
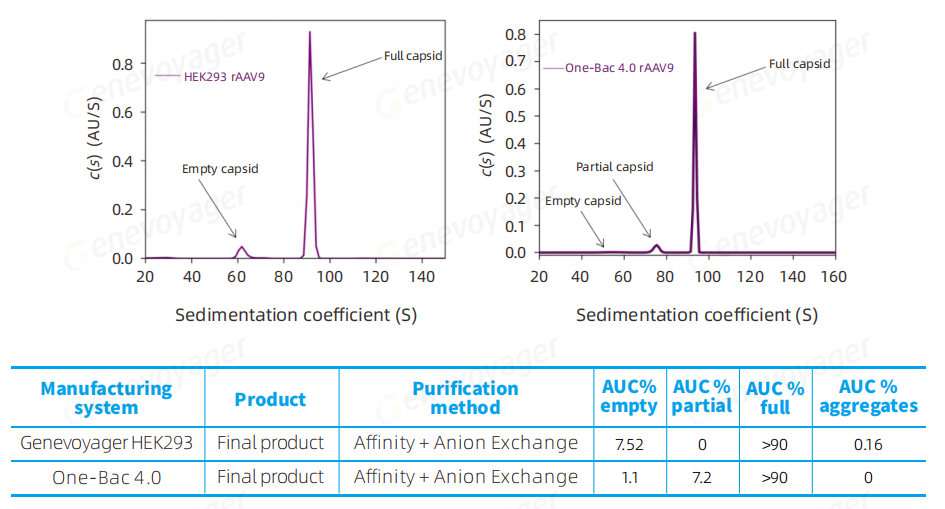
The One-Bac 4.0 system delivers a final product with a full capsid ratio exceeding 90%.
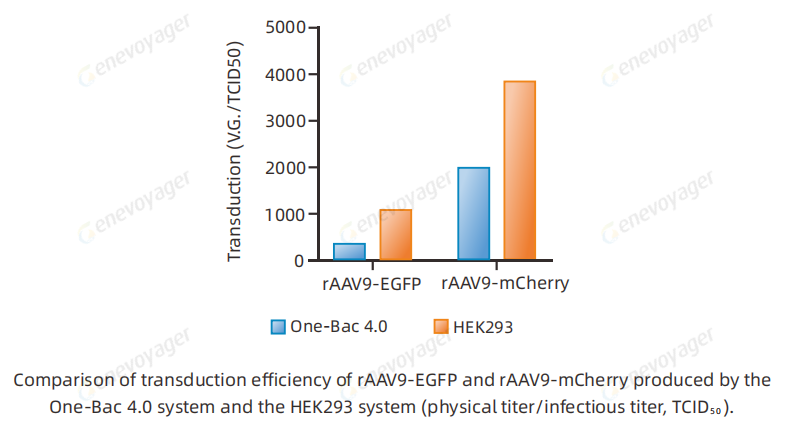
Results from cell and animal experiments demonstrate that One-Bac 4.0-derived AAV exhibits equivalent or higher infectivity compared to HEK293 AAV.
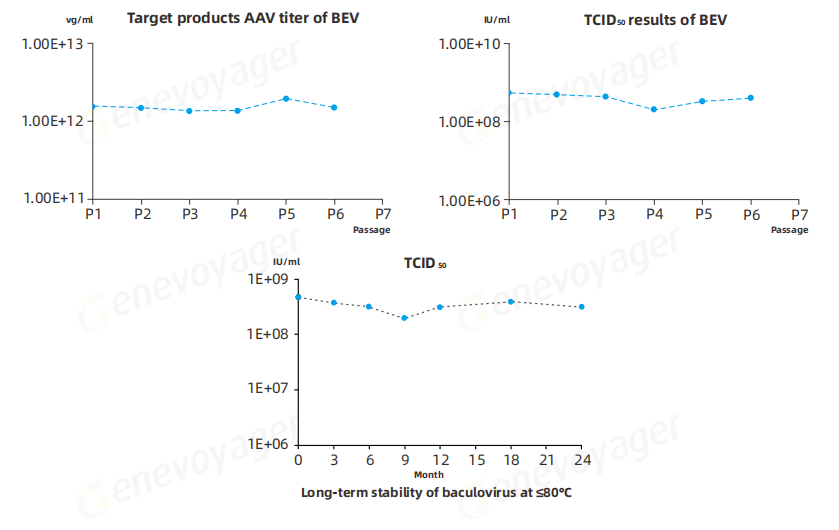
The Baculovirus Expression Vector (BEV) used in the One-Bac 4.0 system demonstrates exceptional stability across multiple passages and after long-term storage at≤-80℃ for over 24 months.This ensures reliable large-scale manufacturing with consistently high AAV yields and robust expression levels suitable for commercial production.
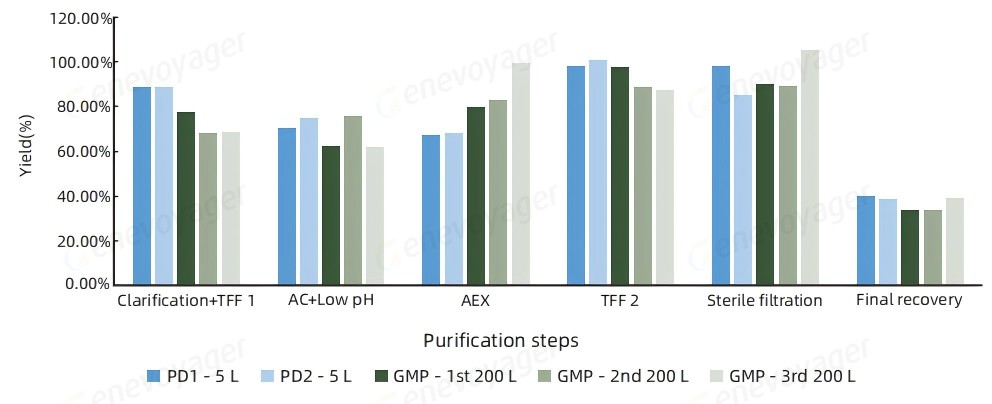
The One-Bac 4.0 system delivers reliable scalability and efficiency for large-scale manufacturing, maintaining a consistent recovery rate of ≥30% from 5L to 200L scale-up.
AAVs produced with the One-Bac 4.0 system show low aggregation even at high concentrations. Stability studies using ddPCR-GOl and SEC-HPLC confirm that product quality is preserved during storage at 4℃ and remains robust after multiple freeze-thaw cycles.
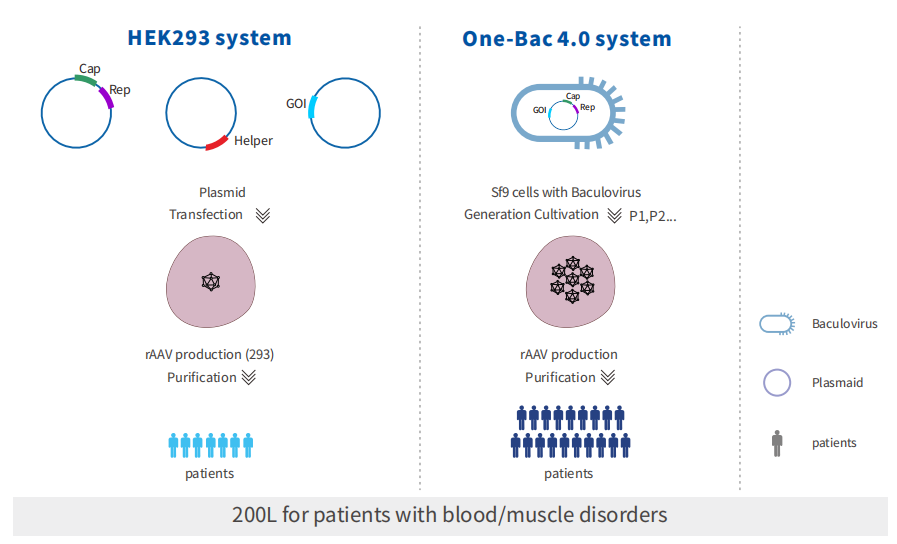
The One-Bac 4.0 system provides a cost-effective solution for AAV production by replacing expensive GMP-grade plasmids and transfection reagents with a streamlined baculovirus-based process, significantly lowering material costs in vector manufacturing.
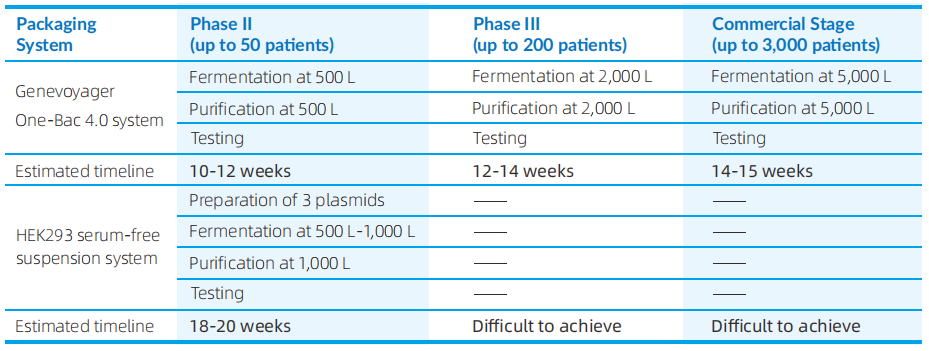
A virus seed bank enables a rapid, streamlined infection process during production. This advantage becomes increasingly significant at larger scales, helping to shorten manufacturing timelines and accelerate delivery.

US: 3675 Market Street, Suite 200, Philadelphia, PA19104 Tel: +1 (215) 205-6963 | +086 027-65023363
E-mail: hui.wang@genevoyager.com / vector@genevoyager.com
China: No128, Guanggu 7th Rd, East Lake High-tech Development Zone, Wuhan, China Tel: 17720522078
E-mail: marketing@genevoyager.com